Beyond Fit For Purpose:
We Make Data Work
For Your Purpose
Real-world evidence research, insights and interdisciplinary support using retrospective and prospective data
Contact us for data source evaluation and study feasibility.
Learn more
Government
CMS Medicare and Medicaid
Veterans Health Administration (VHA)
Department of Defense (DoD)
County Health Rankings and Roadmaps
Commercial Claims
Optum
IQVIA
MarketScan
Humana
Carelon
Premier
Clinical/EMR Sources
Flatiron
Veradigm
QCentrix
IQVIA Oncology
Optum Integrated
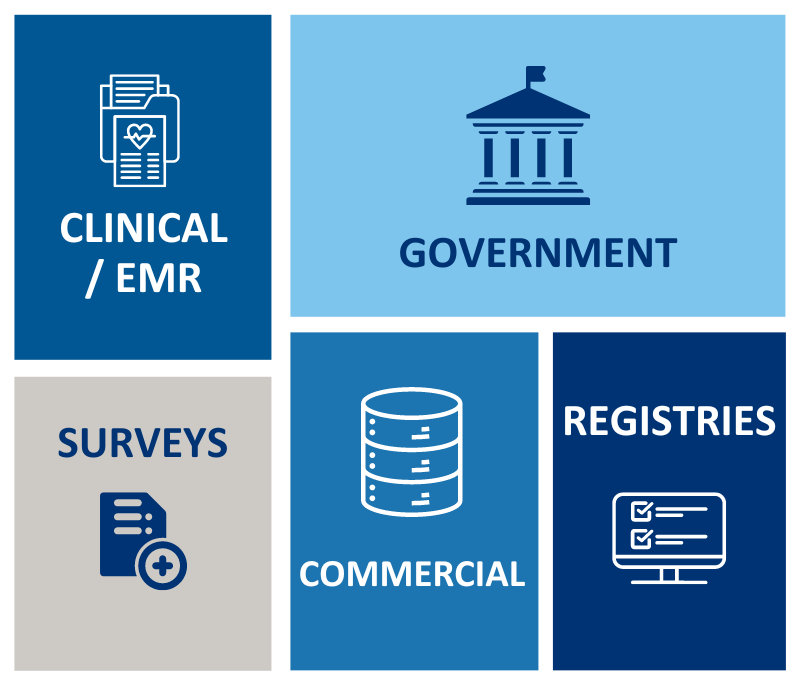
Registries
United States Renal Data System (USRDS)
Surveillance, Epidemiology & End Results (SEER)
University of Michigan Health Registries
Surveys
Agency for Healthcare Research & Quality (AHRQ)
Medical Expenditure Panel Survey (MEPS)
Medicare Current Beneficiary Survey (MCBS)
Ex-US Data
IQVIA Global
Turkey National Data
FinnGen – Finland Healthcare Claims & Registry
Medical Data Vision (MDV) Japan Dataset
JMDC Claims Database (Japan)
Clinical Practice Research Data (CPRD; UK)

- Vital Signs and Demographics – Race/ethnicity, BMI, weight, blood pressure
- Rx Drugs and Treatment Patterns
- Laboratory Test Values – Routine blood panels, urine analysis, acute respiratory screening, more disease-specific measures
- Patient Reported Outcomes (PROs) – Mental health measures, pain scales
- Social Factors and History / Health Maintenance – Family history, alcohol and tobacco use screenings, immunization presence and location
- Social Determinants of Health (SDoH) – Healthcare access, education, employment, finance, housing, societal/cultural
- Healthcare Costs & Utilization – Inpatient, outpatient, ER, pharmacy, hospitalization, length of stay
- Diagnoses & Procedures – Performed and documented procedures
- Other – OTC recommendations, provider referrals, allergies/allergic reactions, mortality